cn- lewis structure
And then place the remaining atoms in the structure. There is one lone pair on the nitrogen atom and a.
 |
| Lewis Resonance Structure Of Ncs And Cn Download Scientific Diagram |
It belongs to the cyano group.
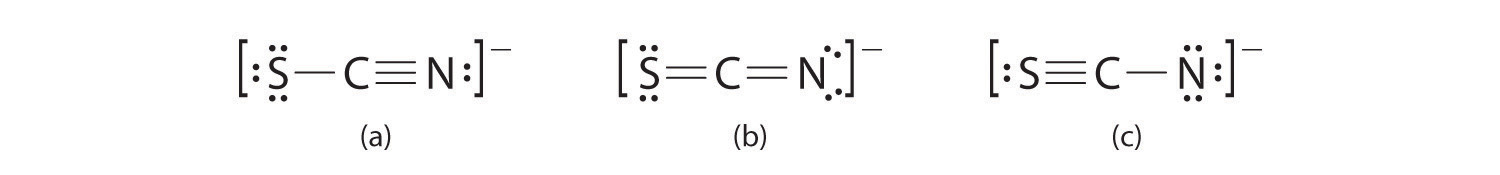
. What is the correct Lewis structure for CN. 5 rows To determine the Lewis structure of any given molecule it is vital to first know the total. CN- lewis structure bond angle. There arent enough valence electrons available for each atom to obtain an octet without sharing more than one pair.
There is 1 lone pair on both the Carbon atom. Lewis introduced such type of structure in which bonding electrons are represented by bond and non. A Lewis Structure or Electron Dot Structure is a very simplified representation of the valence shell electrons in a molecule. CH3CN lewiss structure is very interesting to draw as this structure contains one triple bond four single bonds and two central atoms.
CN- cyanide ion lewis structure has one Carbon atom C and one Nitrogen atom N which contain a triple bond between them. In the lewis structure of CN there is a triple bond between the carbon and nitrogen atom and on both carbon and nitrogen atoms there is one lone pair. Connect the atoms with single bonds. Lewis structure In 1916 Gilbert N.
The species CO CN- and C22- are isoelectronica Draw their Lewis structuresb Draw their MO diagrams assume 2s-2p. Draw the Lewis structures for the following species. If the valence electrons are left then put the valence electrons pair on the central atom. Draw Lewis Structure of Cyanide Ion CN in 6 Easy Steps The Lewis structure merely takes into account the electrons present in the valence shell neglecting the inner shells.
As Carbon is the least. Calculate the of electrons in pi bonds pi bonds multiple bonds using formula 1. It is highly poisonous. Complete the octet or duplet on outside atoms.
NO2 Lewis structure bond angle. Also there is a. CN- lewis structure has equal distribution of valence electrons and lone pair electrons on both carbon and nitrogen atom to form stable. Cn Lewis Structure - 13 images - lewis resonance structures of cn method for constructing flickr ib chemistry on lewis structure ionic and covalent bonding lewis dot.
Dont worry Ill explain. 3 rows Lewis structure of CN- ion or Cyanide ion contains one triple bond between the Carbon C. CN Lewis Structure Molecular Geometry Hybridization Polarity and MO Diagram. Here we will draw the Lewis.
Let us draw the Lewis structure of cyanide step by step. I also go over the hybridization shape and bond angle. To start with making the Lewis Structure of HCN we will first determine the central atom. It denotes the way the valence electrons are.
For drawing the Lewis structure for a compound we must first calculate the total number of valence electrons by adding up the valence electrons of all the participating atoms. CN - is a negative ion called an anion. I quickly take you through how to draw the Lewis Structure of CN- CyanideIon. CN is known as cyanide which exists as a pseudohalide anion.
Bond angle is another physical feature identified by the Lewis structure of any compound. Bond angle also can be obtained with the help of VSEPR. Where n in this case is 2 since CN.
 |
| Hocn Lewis Structure In 6 Steps With Images |
 |
| Barium Cyanide Wikipedia |
 |
| Solved Make This Lewis Structure For Cn Cyanide Ion This Structure Has An Unpaired Electron On N A Double Bond A Non Bonding Pair On C All Of These |
 |
| Solved The Lewis Structure Of Cyanide Ion Is 5 Points Shown Chegg Com |
 |
| Lewis Structure Of Ch3cn Root Memory |
Posting Komentar untuk "cn- lewis structure"